Abstract
Introduction: In sickle cell disease (SCD) mouse models, heme activates toll-like receptor 4 (TLR4) MyD88-dependent signaling and activates NOD-like receptor family pyrin domain containing (NLRP) 3 inflammasome assembly, leading to vascular endothelial activation and vaso-occlusion (VO). In SCD patients, cycles of hemolysis-driven VO contribute to acute and chronic pain/hyperalgesia. Several studies have evaluated non-selective histone deacetylase (HDAC) inhibitors for SCD; however, side-effects have limited clinical advancement. HDAC6 is an attractive target as it exhibits cytoplasmic AND nuclear activity and knockout mice lack hematologic consequences. In the cytoplasm, HDAC6 helps assemble NLRP3 inflammasomes and functions downstream of TLR4 to inhibit signaling. In SCD mouse models, TLR4 signaling is also critical for mechanical hyperalgesia. Intriguingly, in non-SCD hyperalgesia models, HDAC6 inhibition reduces TLR4-mediated mechanical hypersensitivity. We hypothesized that by preventing TLR4/NLRP3 activation in SCD mice, HDAC6 inhibition will prevent VO and pain.
Methods: For in vitro analysis, TLR-4 signaling was stimulated in human umbilical vein endothelial cells (HUVEC) or mouse microglial cells (SIM-A9, ATTC) using 10-20 µM hemin or 10 ng/mL lipopolysaccharide (LPS) ± HDAC6 inhibitor BML-281 (2 µM, Enzo). To assess for pro-adhesive (VCAM-1, P-selectin, VWF) and pro-inflammatory (IL-6, NF-κB, IL-1) gene and protein expression, cell lysates or conditioned media were collected for analysis using qRT-PCR, immunoblotting, and enzyme-linked immunosorbent assay. Immunofluorescence (IF) for P-selectin and VWF was performed on fresh frozen organ sections or cells fixed with 4% paraformaldehyde. To assess for reactive oxygen species generation (ROS), the TMRE assay was used (Abcam). For dorsal skin fold chamber (DSFC) assessment of VO, HbSS Townes mice (n=7 per condition) were subcutaneously injected with either vehicle (DMSO) or BML-281 (1 mg/kg) 24h and 1h prior to DSFC implantation. After DSFC implantation, baseline venules were assessed. To initiate VO, panhematin (3.2 µmole/kg) was infused via tail vein, and at 1-4 hours post-infusion venules were reexamined for stasis. Mice were then sacrificed and organs stored for pathology assessment. For pain studies, HbSS Townes mice (n=6) were assessed for mechanical hyperalgesia for 4 days prior to injections. Only non-hyperalgesic mice were injected subcutaneously with either vehicle (DMSO, n=3) or BML-281 (1 mg/kg, n=3) 24h and 1h prior to panhematin infusion. To induce acute pain, panhematin (3.2 µmole/kg) was infused via tail vein, and 1h, 4h, 24h and 48h post-infusion mechanical hyperalgesia measurements were performed.
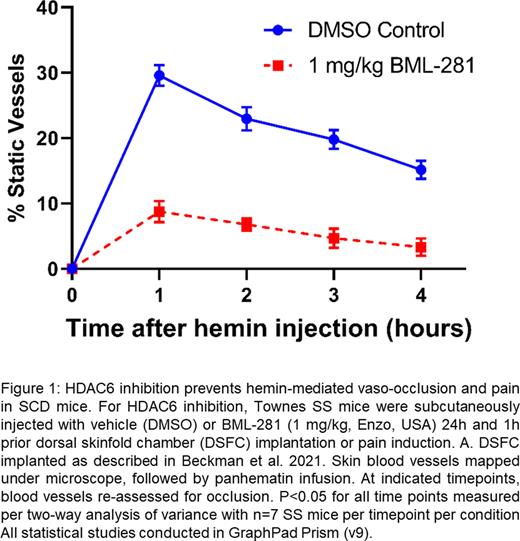
Results: Compared to LPS or hemin treated ECs, addition of 2 µM BML-281 prevented upregulation of , VCAM-1, P-selectin and E-selection mRNA, this was inhibited by the addition of 2 µM BML-281. Likewise, compared to hemin-treated ECs, BML-281+hemin-treated ECs had reduced pro-adhesive VCAM-1 and E-selectin protein expression. In IF studies, ECs treated with BML-281+hemin had reduced VWF expression and P-selectin release. Hemin-treated HUVEC exhibit increased NLRP3-mediated ROS production, which BML-281 treatments was significantly decreased. In DSFC studies, compared to vehicle-treated mice, BML-281-treated SS Townes mice had significantly fewer static vessels after panhematin infusion (Fig 1). In pathology assessment, BML-281 treated mice, showed reduced lung VWF and P-selectin vessel deposition. To assess neuroinflammation, mouse microglial cells were treated with LPS. Compared to LPS alone, BML-281 treatment significantly reduced IL-6 secretion and NLRP3 mRNA upregulation. Importantly, in in vivo pain assessments, compared to vehicle-treated SS-Townes mice at 1 h and 4 h post-hemin infusion, BML-281-treated SS-Townes mice exhibited significantly less mechanical hyperalgesia.
Conclusion: Our studies show that HDAC6 inhibition prevented hemin-mediated VO, potentially through reduction in hemin-mediated ROS and TLR4 driven pro-adhesive endothelial activation. Furthermore, HDAC6 inhibition prevented hemin-mediated pro-inflammatory microglial activation and reduced hemin-mediated acute pain stimulation. Collectively, these studies suggest that selective HDAC6 inhibition may be a strategy to reduce acute inflammation and pain in SCD.
Disclosures
Belcher:Omeros: Research Funding; Mitobridge-Astellas: Research Funding; CSL-Behring: Research Funding. Vercellotti:Omeros: Research Funding; Mitobridge-Astellas: Research Funding; CSL-Behring: Research Funding. Beckman:Bayer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal